

|


The UK HARP-III trial is investigating whether a new drug (sacubitril/valsartan, formerly known as LCZ696) has the potential to protect kidneys better than current standard treatment. Chronic kidney disease affects about 1 in 10 adults. The illness can worsen over time. This means that some people eventually need to have dialysis or a kidney transplant. There are treatments that can slow the rate of kidney decline. However, despite such treatment some people still need transplantation or dialysis. Two commonly-used treatments are: 'ACE inhibitors' (you may be familiar with them as their drug names end with the letters '-pril') and Angiotensin receptor blockers (the drug names end in '-sartan').
Sacubitril/valsartan is a new treatment which has two actions: one half of the drug is the same as an angiotensin receptor blocker (valsartan). The other half of the drug is a 'neprilysin inhibitor' (sacubitril) which prevents the breakdown of certain proteins in the blood. Blocking their breakdown might slow the progression of kidney damage and delay the need for dialysis and transplant. These drugs may also benefit the heart and blood circulation.
The UK HARP-III trial is registered with the following bodies:
• ISRCTN (International Standard Randomised Controlled Trial Number): 11958993
• EU Clinical Trials Register: 2013-004205-89
• UKCRN (UK Clinical Research Network): 15842
The study has now completed. Please visit the News section for details of the results presented at the American Society of Nephrology on 3rd November 2017.
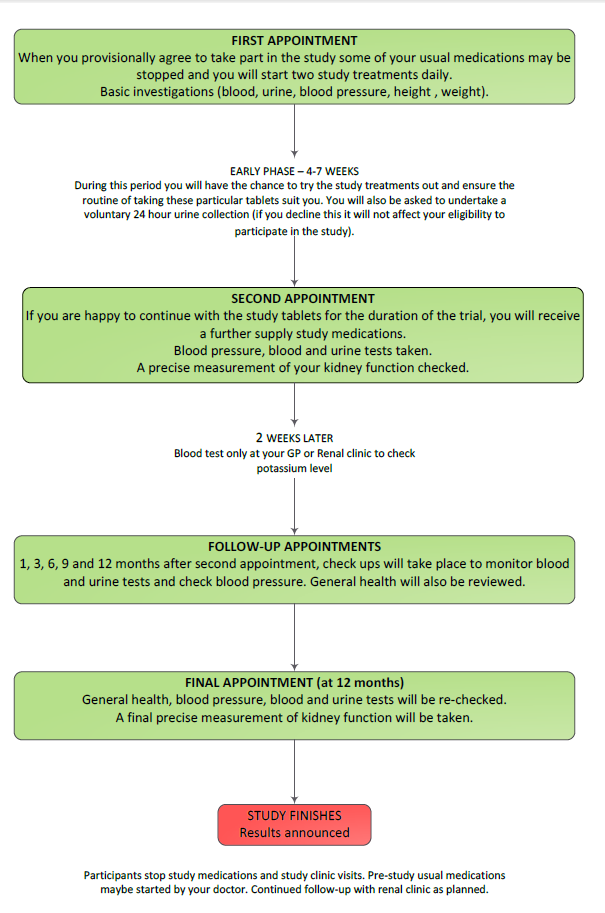
You will need to participate in UK HARP-III for about 13 months. You will be asked to attend about seven hospital appointments in total, some of which may coincide with your routine renal outpatient appointments. Participants are selected randomly (by chance) to receive either active LCZ696 and placebo irbesartan, or placebo LCZ696 and active irbesartan.
What is the purpose of the study?
The study is called the UK HARP-III trial. It is investigating whether a new drug (LCZ696) has the potential to protect kidneys better than current standard treatment.
Chronic kidney disease affects about 1 in 10 adults. The illness can worsen over time. This means that some people eventually need to have dialysis or a kidney transplant.
There are treatments that can slow the rate of kidney decline. However, despite such treatment some people still need transplantation or dialysis. Two commonly-used treatments are:
• “ACE inhibitors” (you may be familiar with them as their drug names end with the letters “-pril”. And,
• Angiotensin receptor blockers (the drug names end in “-sartan”)
LCZ696 is a new treatment which has two actions: one half of the drug is the same as an angiotensin receptor blocker (valsartan). The other half of the drug is a “neprilysin inhibitor” which prevents the breakdown of certain proteins in the blood. Blocking their breakdown might slow the progression of kidney damage and delay the need for dialysis and transplant. These drugs may also benefit the heart and blood circulation.
^Back to FAQDo I have to take part?
No. Participation is entirely up to you. If you agree to take part we will ask you to sign a form to show that you have consented. You are free to withdraw at any time, without giving a reason. This will not affect the standard of care you receive.
^Back to FAQWho is running and who is funding the study?
UK HARP-III is being led by experienced medical scientists at the University of Oxford who carried out the important Study of Heart and Renal Protection (SHARP). This study showed the benefits of lowering “bad” cholesterol in people with chronic kidney disease and resulted in changes to medical practice around the world. Treatment for this study is provided free by Novartis (a pharmaceutical company), which also contributes to the cost of running of the study, by a grant to the University of Oxford. The results will be analysed by scientists at the University of Oxford independently of Novartis.
^Back to FAQWhat will happen to me if I take part?
You will need to participate in UK HARP-III for about 13 months. You will be asked to attend about seven hospital appointments, some of which may coincide with your routine renal outpatient appointments. In addition, we will measure your kidney function very precisely on two occasions during the study.
Getting started At your first visit to the HARP-III clinic a trained researcher (usually a nurse) will ask you about your medical history. They will explain the study to you and you will be given plenty of opportunity to ask questions. The researcher will take your blood pressure and a sample of blood and urine. If you are interested in the study, you will be asked to sign a form agreeing to take part. We will write to your GP about your participation in the study. You will then be provided with a supply of the study tablets and asked to take two a day. This visit will take about 45 minutes.
You may be asked to stop some of your current blood pressure treatment (because the study treatment will replace them). Over the course of the next few weeks you will have the chance to try out the study tablets. This will allow you and the UK HARP-III doctors and nurses to be sure the routine of taking these particular tablets agree with you. You will be given a container and asked to collect a sample of your urine on the morning of your next visit and bring it with you to the study clinic. Towards the end of this period you may also be asked to collect your urine for 24 hours, but this optional and you can still participate even if you don’t want to do this.
After 4 to 7 weeks After at least 4 weeks of taking the study tablets you will be asked to attend a second appointment to see if you would like to continue. We will measure your kidney function very precisely (see below). Your blood pressure will be checked and we will ask for another blood sample. Your height and weight will also be recorded at this visit. If you have had no problems with the study treatments during the first few weeks and are happy to continue, you will be asked to commit to the study for another 12 months. This visit will take about 30 minutes.
At this appointment your treatment for the rest of the study will be decided “at random” (like the toss of a coin). You will be given two drugs (one, an active treatment and the other a ‘dummy’ placebo). These will be either active LCZ696 and placebo irbesartan, or placebo LCZ696 and active irbesartan. Irbesartan is an angiotensin receptor blocker and is commonly used to treat kidney disease. The initial dose is one tablet of each treatment once daily.
You have as much chance of receiving LCZ696, as you do of receiving the standard treatment, irbesartan. You will not know which treatment you receive, nor will your GP or the UK HARP-III staff. However, this information would be made available to your doctor and other medical staff if this was medically necessary.
Further visits You will be asked to have a blood test after about two weeks to check your potassium level. This can be done at your GP surgery or your renal clinic. The dose of your study treatment will then increase to the full dose (two tablets of active treatment and two tablets of placebo once daily).
You will be asked to attend five further appointments (about 1, 3, 6, 9 and 12 months later) to see how you are getting on. You will be asked to bring a urine sample collected on the morning of each of these visits to the clinic (in a container provided at the previous visit). Your blood pressure will be checked and a blood sample will be taken at each visit. At the final visit you will also have a second precise measurement of your kidney function. Each visit will last about 30 minutes.
In the unlikely event that your blood test results are of concern (for example, if your potassium level was high) you may be asked to attend an extra visit. We would repeat the blood test and further checks would be done. With regular check-ups from the UK HARP-III specialist nursing team, you can be assured of the best possible follow-up care and attention. If any problems emerge for you while you are on the study, your consultant and GP will be informed.
^Back to FAQBlood and urine samples
The blood and urine samples that you provide will be tested locally to check that the study treatments are not having any adverse effects. We need about 4 teaspoons of blood on each occasion. Some of the samples will also be sent to the central laboratory in Oxford University. This allows us to see whether the effects of the treatments vary between different types of people taking part. We will also look to see if the treatments affect other markers of kidney function.
We will also ask for permission to store your blood and urine samples long-term. These samples will not have your name on. This will help with other kidney studies and research into other diseases.
^Back to FAQMeasuring kidney function
In routine clinical practice, your doctors estimate kidney function by looking at the level of a substance called creatinine in the blood. This is sufficient for clinical purposes, but in the UK HARP-III study we need to measure kidney function more precisely. This involves having an injection of a very small amount of a substance (which in some hospitals may be radioactive) and then a number of blood samples in the 4-5 hours that follow (this may take longer at certain hospitals). This allows us to measure how quickly your kidneys remove this substance from your blood. This test is routinely used in the NHS when kidney function needs to be measured precisely. If used, the amount of radioactivity is very small (equivalent to about ten days of normal background radiation or less in the UK), so represents a negligible risk to your health.
^Back to FAQTravel expenses
We are happy to reimburse reasonable expenses for travelling to your UK HARP-III appointments. Please make sure you ask about this at the clinic.
^Back to FAQWhat will I have to do?
For UK HARP-III to produce the best results, it is important that people stay in the study for its duration if possible. You will need to attend the UK HARP-III clinic seven times during the 13-14 months of the study. Extra appointments can also be arranged if you are worried about the study tablets. However, you can withdraw from the study at any time.
You will be asked to take either a drug called irbesartan or the new treatment, LCZ696. Scientists do not know which treatment is best. You may be asked to stop some of your current medications because the study treatment will replace them. We will discuss this with you at your appointment.
We will ask you to provide blood and urine samples and to give permission for them to be stored for future tests. We will also ask about your health. Your blood pressure will be measured at every visit. At some visits extra measurements will be taken, including your height and weight.
^Back to FAQWhat are the benefits of taking part in this study?
You may be helping yourself, but you will most certainly be helping doctors and scientists improve treatment for people who have chronic kidney disease and who may be at risk of needing dialysis or a transplant. If successful, results from this study will help to design a larger trial of LCZ696 which could reliably show whether LCZ696 is better than current treatment in slowing the progression of chronic kidney disease.
^Back to FAQAre there any risks?
Most treatments have side-effects which some people may experience and others do not. If you do experience any side effects while on the UK HARP-III study they will be noted, so that scientists can learn from you. You can withdraw from the study if you wish.
• Irbesartan is generally very well-tolerated. It has been tested in thousands of people and is taken by hundreds of thousands of people worldwide. It lowers blood pressure so it can cause dizziness. Other side-effects include nausea, muscle pain and fatigue. Like all “angiotensin receptor blockers” it can raise potassium levels in the blood and you will be monitored for this.
• LCZ696 is an unlicensed drug and is being tested in this study. Over 8,000 people have taken LCZ696 in other trials and it is generally well-tolerated. It also lowers blood pressure so can cause dizziness and fatigue. Rarely it may cause swelling of the mouth and face (angioedema), but it does not appear to do this more frequently than “ACE inhibitors” which are a very commonly used medication in people with chronic kidney disease. It is very important that you do not take LCZ696 with an ACE inhibitor (e.g. ramipril, lisinopril). The treatment can raise potassium levels in the blood and you will be monitored for this. One patient who received LCZ696 had an allergic reaction which included abnormal liver function tests. At this stage, scientists cannot rule out the possibility of there being side effects (such as diarrhoea or muscle pains), or effects on other blood tests.
Throughout the study you would be carefully monitored by our nursing team for possible side effects. At every visit, the study staff would discuss any new information about the drug with you.
There is nothing to suggest that stopping the tablets will cause you harm. If you do experience side effects, you may choose, or be advised by your doctor, to stop the tablets provided by the study.
If you do experience unexpected symptoms after joining the study you can contact your UK HARP-III nurse, or a study doctor on
Freefone 0800 585323 (available 24 hours a day, 7 days a week).
What are the other possible disadvantages of taking part?
The study includes two precise measurements of your kidney function which may involve an injection of a small amount of radioactive material. The dose of radioactivity is small (equivalent to ten days of natural background radiation or less in the UK) and poses a negligible risk to health.
Before participating you should check whether doing so will affect any insurance that you have and seek advice if necessary.
For women Irbesartan should not be taken by pregnant or breast-feeding women. The effects of LCZ696 on pregnancy and the unborn child and breast-feeding are not known. Women who could become pregnant must use effective contraception during the course of this study. If you become pregnant during the trial (or wish to do so), you should tell your study nurse or doctor immediately so appropriate action can been discussed.
^Back to FAQWhat happens when the study stops?
You and your doctors will be informed of the study results when they become available. LCZ696 does not have a license in the UK currently so it will not be available once the study is complete. However this study will help design a larger trial of LCZ696 in people with chronic kidney disease which could lead to it becoming available. At the end of the study you will go back to any treatment that you stopped. Your doctor will advise you about this.
^Back to FAQWhat if there is a problem?
If you have any concerns about any possible side-effects of treatment or any complaint about the way you have been dealt with during the study, please call the study team on Freefone 0800 585323.
^Back to FAQPATIENTS WITH PROTEINURIC CHRONIC KIDNEY DISEASE (CKD) ARE AT RISK OF PROGRESSION TO END-STAGE RENAL DISEASE
CKD is often a progressive condition and proteinuria is a significant risk factor for more rapid progression. Although most groups of patients with CKD are more likely to die than reach end-stage renal disease (ESRD), this is still a highly desirable outcome to avoid due to the effect on quality of life and associated costs to healthcare providers. Blockade of the renin-angiotensin system with angiotensin enzyme converting inhibitors (ACEi) or angiotensin receptor blockers (ARB) are proven to slow the rate of progression in proteinuric nephropathies, but a substantial residual risk of ESRD remains. Despite initial enthusiasm for combination therapy (e.g. ACEi plus ARB or ACEi plus direct renin inhibition) there is no evidence that this retards renal progression and may in fact be hazardous. There is therefore a need for treatments that can be safely added to current standard treatments to slow the progression to ESRD in patients with CKD.
CARDIOVASCULAR DISEASE IS THE COMMONEST CAUSE OF DEATH IN PATIENTS WITH CHRONIC KIDNEY DISEASE
Patients with chronic kidney disease are at substantially increased risk of premature cardiovascular morbidity and mortality compared with the general population, but there is little reliable information about treatments that are effective for its prevention. Cardiovascular disease (CVD) in patients with CKD is complex and multifactorial, and differs in both aetiology and manifestation from the general population. Recently, lowering LDL cholesterol was shown to reduce the risk of major atherosclerotic events among patients with CKD, but most CVD is not atherosclerotic in CKD and therefore treatments are needed which can reduce this risk of this CVD.
LCZ696 HAS PROPERTIES THAT COULD REDUCE BOTH RENAL PROGRESSION AND CARDIOVASCULAR DISEASE
Neprilysin (also known as neutral endopeptidase) degrades natriuretic and other vasodilatory peptides and therefore neprilysin inhibition increases concentrations of these peptides and can lower blood pressure (in combination with ACEi or ARB). Omapatrilat inhibited neprilysin but caused unacceptable angioedema when combined with ACEi. However, some data suggested it was renoprotective. LCZ696 (sacubitril valsartan [Entresto]) is a first-in-class ARNI (angiotensin receptor-neprilysin inhibitor) which appears to be well-tolerated in people without CKD. The anti-fibrotic and anti-inflammatory effects of LCZ696 may be beneficial both in terms of reducing renal progression and reducing CVD events.
This study will include at least 400 participants aged at least 18 years with CKD with an eGFR ≥20 <60 mL/min/1.73m2 (including about 180 participants with an eGFR <45 mL/min/1.73m2).
Primary aim
The primary aim is to assess the difference in the change in measured glomerular filtration rate (mGFR; measured by 51Chromium-EDTA clearance ) from baseline to 12 months between participants allocated LCZ696 versus those allocated irbesartan control.
Secondary and tertiary aims
Secondary aims include assessment of the effects of LCZ696 on albuminuria and the population pharmacokinetics of LCZ696 and its metabolites. Tertiary aims include assessment of the tolerability and safety of LCZ696 and its effects on various biomarkers of kidney damage and blood pressure.
POTENTIALLY ELIGIBLE
• patients with established chronic kidney disease, defined by:
- an estimated glomerular filtration rate ≥20 <45 mL/min/1.73m2; OR
- an estimated glomerular filtration rate ≥45 <60 mL/min/1.73m2; AND albumin:creatinine ratio >20 mg/mmol (or protein:creatinine ratio >30 mg/mmol)
• age ≥18 years
• no definite indication for, or contraindication to, LCZ696
IDENTIFICATION AND INVITATION
• potentially eligible patients identified from local hospital database
• patient invited to attend Screening clinic appointment in local study clinic
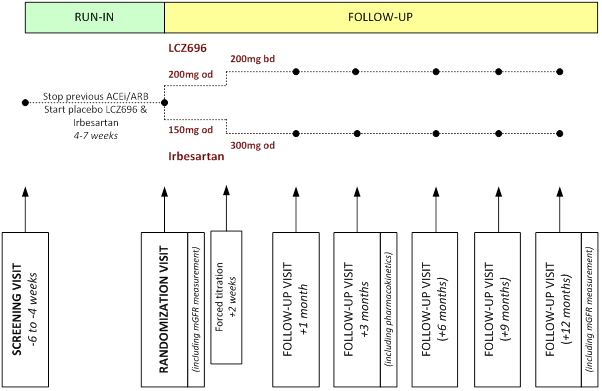
SCREENING VISIT (-7 to -4 WEEKS)
• medical history, relevant current treatment and other eligibility factors recorded
• written informed consent sought from eligible and willing patients
• any ACEi, ARB or direct renin inhibitor (DRI) therapy stopped
RUN-IN PHASE
• "wash out" of prior ACEi or ARB therapy
• any participants unable to tolerate placebo LCZ696 withdrawn from Run-in
• permission sought from Local Lead Investigator (LLI) to randomize eligible participant and GP informed
• GFR measured by 51Cr-EDTA at least 4 weeks after Screening visit
• optional 24 hour urine collection (to measure sodium excretion)
RANDOMIZATION VISIT (0 WEEKS)
• final check of eligibility and adherence
• non-study treatment and adverse events during Run-in recorded
• mean sitting blood pressure recorded and baseline blood and urine samples collected
• randomly allocated to LCZ696 versus irbesartan
• participant's nephrologist and general practitioner informed of randomization
FOLLOW-UP (+2 weeks and +1, + 3, and +6, +9 and +12 months)
• check of electrolytes at +2 weeks prior to forced titration
• non-study treatment, adherence, adverse events assessed at study clinic visit
• mean sitting blood pressure recorded
• blood and urine samples collected (including pharmacokinetic samples at +3 months)
• further treatment provided to participant as required and specifically at the 3, 6 and 9 month visits
• at or just prior to +12 month visit GFR measured by 51Cr-EDTA
• ARB therapy contraindicated e.g. bilateral renal artery stenosis
• Known intolerance of ARB
• Current treatment with aliskiren
• Mean systolic blood pressure >180 mmHg at screening visit (or investigator unwilling to withdraw ACEi or ARB for another reason)
• Serum potassium >5.5 mmol/L
• Presence of nephrotic syndrome (i.e. urine protein:creatinine ratio >350 mg/mmol [or albumin:creatinine ratio >300 mg/mmol] AND serum albumin <30 g/L) or currently receiving immunosuppression for nephrotic syndrome
• Functioning renal transplant
• Acute coronary syndrome, stroke or transient ischaemic attack in 3 months prior to Screening
• Known chronic liver disease or ALT/AST >2x ULN at Screening
• History of angioedema (drug-related or otherwise)
• Use of unlicensed investigational medicinal product in previous month
• Pregnancy or women with child-bearing potential (refusing a reliable method of contraception)
• Medical history that might limit the patient’s ability to take study treatments for the duration of the study (e.g. severe respiratory disease, or recent history of alcohol or substance misuse or history of cancer or evidence of spread in last 5 years other than non-melanoma skin cancer)
 Chief Investigator
Chief Investigator
 Clinical Research Fellow
Clinical Research Fellow
 Clinical Research Fellow
Clinical Research Fellow
 Clinical Research Fellow
Clinical Research Fellow
 Senior Study Administrator
Senior Study Administrator
 Study Administrator
Study Administrator
 Study Administrator
Study Administrator
 Senior Study Administrator
Senior Study Administrator
 Senior Study Administrator
Senior Study Administrator
 International Nursing Coordinator
International Nursing Coordinator
The results of the UK HARP-III trial were made public on 3rd November 2017. Here follows a brief summary.
414 participants were randomized into the UK HARP-III trial. Their average age was 62 years and about 70% were male. The average estimated glomerular filtration rate (eGFR) was 35 mL/min/1.73m2 and median urine albumin:creatinine ratio was about 50 mg/mmol. The study population had a wide variety of causes of kidney disease.
During the study, 20% of participants stopped their assigned study treatment with a similar proportion in both groups.
The primary outcome (measured GFR) did not differ between the two groups: the difference in means was -0.1 mL/min/1.73m2. The results were similar for estimated GFR (based on the blood tests done at each study visit).
Sacubitril/valsartan caused a small (but not statistically-significant) reduction in albuminuria. It did reduce blood pressure more than irbesartan (by about 5/2 mmHg).
The number of adverse events was similar between the two groups, showing that the safety and tolerability of sacubitril/valsartan is similar to that of irbesartan.
More detail about the results can be found on the poster that was presented at the American Society of Nephrology on 3rd November 2017. The results will be published in full in a medical journal in 2018.
24-hour Freefone service: 0800 585323
By post: UK HARP-III, Clinical Trial Service Unit (CTSU), Richard Doll Building, University of Oxford, Roosevelt Drive, OXFORD, OX3 7LF
By email: harp3@ndph.ox.ac.uk